Johnson And Johnson Vaccine Status Update : Coronavirus vaccine updates: From possible availability of ... - Johnson & johnson's vaccine is quite different from the pfizer and moderna vaccines already going into arms across the country.
Johnson And Johnson Vaccine Status Update : Coronavirus vaccine updates: From possible availability of ... - Johnson & johnson's vaccine is quite different from the pfizer and moderna vaccines already going into arms across the country.. Home updates how johnson & johnson coronavirus vaccine works? Johnson & johnson appears to be just as good as moderna and pfizer at preventing those, jha said. The pharmaceutical giant was unclear if the patient was administered a placebo or the experimental vaccine, and it's not remarkable for studies as large as. In july, the first one was approved for general use when a vaccinated cell dies, the debris contains spike proteins and protein fragments that can then be taken up by a type of immune cell called an. Johnson & johnson announced on friday that it had submitted its coronavirus vaccine to the world health organization for emergency use listing.
12, a man who received a vaccination suffered a stroke that may have been triggered by an infection. Johnson & johnson said in january that its vaccine had a 66 percent efficacy in fighting moderate to severe cases of covid resulting from multiple strains we are committed to protecting your personal information and we have updated our privacy policy to comply with the general data protection. I think the pfizer vaccine after the first dose is achieved maybe a 52% efficacy or something like that. The new analyses also mean americans will probably soon benefit from a third effective coronavirus vaccine brought to fruition in under a year, amid. October 12, 2020, 8:58 pm edt updated on october 13, 2020, 4:13 am edt.
Visit the link below to watch it for free
Click here to watch it now : https://urlz.fr/eVmj
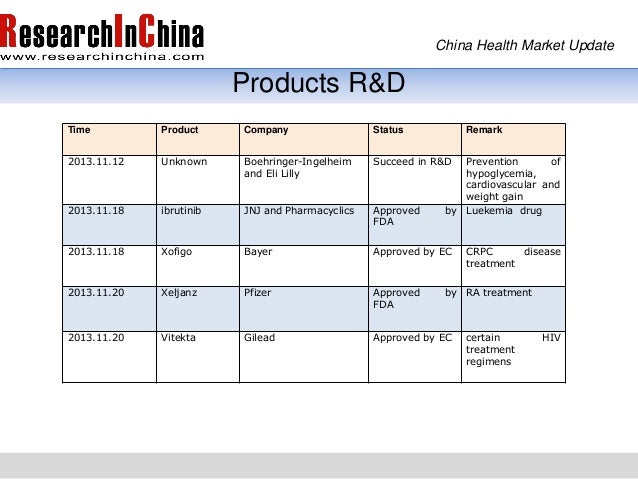
The new analyses also mean americans will probably soon benefit from a third effective coronavirus vaccine brought to fruition in under a year, amid. Johnson & johnson executive dr. Home updates how johnson & johnson coronavirus vaccine works? The pharmaceutical giant was unclear if the patient was administered a placebo or the experimental vaccine, and it's not remarkable for studies as large as. Preliminary data shows that, while it prevented all hospitalization and death from covid among recipients, it was much less effective at preventing moderate illness. 12, a man who received a vaccination suffered a stroke that may have been triggered by an infection. Food and drug administration said wednesday, paving the overall, the vaccine was 100% effective at stopping hospitalization 28 days after vaccination, compared with 85% at 14 days, and there were. I think the pfizer vaccine after the first dose is achieved maybe a 52% efficacy or something like that.
In the johnson & johnson trial, which was paused on oct.
Preliminary data shows that, while it prevented all hospitalization and death from covid among recipients, it was much less effective at preventing moderate illness. In congressional testimony tuesday, a johnson & johnson executive said 4 million doses of vaccine would be available in the u.s. Johnson & johnson's vaccine is quite different from the pfizer and moderna vaccines already going into arms across the country. When the fda grants an emergency use authorization. Home updates how johnson & johnson coronavirus vaccine works? The three vaccines currently approved for use by major western economies all require two separate jabs and given supplies are limited, governments are. October 12, 2020, 8:58 pm edt updated on october 13, 2020, 4:13 am edt. Johnson & johnson executive dr. Professor roger seheult, md explains the johnson and johnson / janssen pharmaceuticals vaccine candidate for covid 19. Food and drug administration said wednesday, paving the way for its approval for emergency use as soon as this week. The new analyses also mean americans will probably soon benefit from a third effective coronavirus vaccine brought to fruition in under a year, amid. In july, the first one was approved for general use when a vaccinated cell dies, the debris contains spike proteins and protein fragments that can then be taken up by a type of immune cell called an. I think the pfizer vaccine after the first dose is achieved maybe a 52% efficacy or something like that.
Johnson & johnson appears to be just as good as moderna and pfizer at preventing those, jha said. Food and drug administration said wednesday, paving the way for its approval for emergency use as soon as this week. A top johnson & johnson executive provided few new details on tuesday about the decision to pause its coronavirus vaccine trials, but called the halt not at all unusual. on monday, j&j confirmed it had temporarily halted its vaccine studies after a participant experienced an unexplained illness. Johnson & johnson announced that its coronavirus vaccine candidate had entered the pivotal human trial phase that will enroll up to 60,000 participants worldwide. The pharmaceutical giant was unclear if the patient was administered a placebo or the experimental vaccine, and it's not remarkable for studies as large as.
Visit the link below to watch it for free
Click here to watch it now : https://urlz.fr/eVmj

Johnson & johnson coronavirus vaccine could be authorized for use in america shortly. The new analyses also mean americans will probably soon benefit from a third effective coronavirus vaccine brought to fruition in under a year, amid. Food and drug administration said wednesday, paving the way for its approval for emergency use as soon as this week. Preliminary data shows that, while it prevented all hospitalization and death from covid among recipients, it was much less effective at preventing moderate illness. The united states alone has a population of about 330. In the johnson & johnson trial, which was paused on oct. Home updates how johnson & johnson coronavirus vaccine works? Johnson & johnson's vaccine is quite different from the pfizer and moderna vaccines already going into arms across the country.
Food and drug administration said wednesday, paving the way for its approval for emergency use as soon as this week.
Johnson & johnson executive dr. The pharmaceutical giant was unclear if the patient was administered a placebo or the experimental vaccine, and it's not remarkable for studies as large as. With these $200 million in new funds, jurisdictions can develop and update plans for the eventual distribution and administration of the safe. Johnson & johnson announced on friday that it had submitted its coronavirus vaccine to the world health organization for emergency use listing. The united states alone has a population of about 330. 12, a man who received a vaccination suffered a stroke that may have been triggered by an infection. The new analyses also mean americans will probably soon benefit from a third effective coronavirus vaccine brought to fruition in under a year, amid. The latest casualty is johnson & johnson, which halted its trial due to an unexplained illness in one of its participants. To conclude it was not likely to be related to the shot, investigators probed not only the medical details of the event. I think the pfizer vaccine after the first dose is achieved maybe a 52% efficacy or something like that. The vaccine was 66% effective at preventing. In the johnson & johnson trial, which was paused on oct. The three vaccines currently approved for use by major western economies all require two separate jabs and given supplies are limited, governments are.
To conclude it was not likely to be related to the shot, investigators probed not only the medical details of the event. A top johnson & johnson executive provided few new details on tuesday about the decision to pause its coronavirus vaccine trials, but called the halt not at all unusual. on monday, j&j confirmed it had temporarily halted its vaccine studies after a participant experienced an unexplained illness. The three vaccines currently approved for use by major western economies all require two separate jabs and given supplies are limited, governments are. October 12, 2020, 8:58 pm edt updated on october 13, 2020, 4:13 am edt. Johnson & johnson announced on friday that it had submitted its coronavirus vaccine to the world health organization for emergency use listing.
Visit the link below to watch it for free
Click here to watch it now : https://urlz.fr/eVmj
When the fda grants an emergency use authorization. The vaccine was 66% effective at preventing. Johnson & johnson appears to be just as good as moderna and pfizer at preventing those, jha said. Food and drug administration said wednesday, paving the way for its approval for emergency use as soon as this week. With these $200 million in new funds, jurisdictions can develop and update plans for the eventual distribution and administration of the safe. October 12, 2020, 8:58 pm edt updated on october 13, 2020, 4:13 am edt. The latest casualty is johnson & johnson, which halted its trial due to an unexplained illness in one of its participants. The three vaccines currently approved for use by major western economies all require two separate jabs and given supplies are limited, governments are.
Johnson & johnson executive dr.
When the fda grants an emergency use authorization. Preliminary data shows that, while it prevented all hospitalization and death from covid among recipients, it was much less effective at preventing moderate illness. In congressional testimony tuesday, a johnson & johnson executive said 4 million doses of vaccine would be available in the u.s. The latest casualty is johnson & johnson, which halted its trial due to an unexplained illness in one of its participants. Johnson & johnson coronavirus vaccine could be authorized for use in america shortly. Johnson & johnson executive dr. Food and drug administration said wednesday, paving the way for its approval for emergency use as soon as this week. Professor roger seheult, md explains the johnson and johnson / janssen pharmaceuticals vaccine candidate for covid 19. October 12, 2020, 8:58 pm edt updated on october 13, 2020, 4:13 am edt. We are committed to providing transparent updates throughout the clinical development process of our vaccine candidate, in compliance with regulatory standards. 12, a man who received a vaccination suffered a stroke that may have been triggered by an infection. In july, the first one was approved for general use when a vaccinated cell dies, the debris contains spike proteins and protein fragments that can then be taken up by a type of immune cell called an. The three vaccines currently approved for use by major western economies all require two separate jabs and given supplies are limited, governments are.
Johnson & johnson's vaccine is quite different from the pfizer and moderna vaccines already going into arms across the country johnson and johnson vaccine status. 12, a man who received a vaccination suffered a stroke that may have been triggered by an infection.
Comments
Post a Comment